Fundamentals of the Stabilization of Soils With Lime
Dallas N. Little
Professor of Civil Engineering
Texas A&M UniversitySummary
Lime is an effective stabilizer for a wide range of soils. Actually, two phases of stabilization occur in a lime-soil system. The first involves the practically immediate reactions of cation exchange and flocculation-agglomeration. These reactions accur to some extent with all fine-grained soils. Due to textural changes caused by these reactions within the soil., the strength and moisture stability of these soils is improved. These improvements are reflected in improved workability, imediate strength improvement and reduced swell-susceptibility.
Pozzolanically induced long-term strength gain is more capricious as its succes depends upon a cooperative reaction between the lime and the clay as well as the mineralogy of the clay. Many clays are reactive and upon lime stabilization their strength may easily triple or quadruple. In some instances strengths have improved by an order of 10 or more.
Lime, the versatile soil stabilizer, should be considered with all soils when the plasticity index exceeds 10 percent and the percent of soil smaller than the number 200 sieve exceeds 25 percent.
Need for Stabilization
Soils often require stabilization to add mechanical stability, to improve durability or to alter their volume change potential. The mostwidely recognized form of stabilization is compaction, which improves the machanical stability of virtually any soil. However, compaction alone is often not enough. Thsi is especially true with fine-grained, cohesive soils.
Plastic clays pose a unique problem to the enginer. Their consistency varies over a very wide range. This consistency is directly related to the availability of water. The ability of clays to take-on and lose water is a function of their morphology and mineralogical nature.
Lime (either quicklime or hydrated lime, in both high calcium anddolomitic types) is an effective stabilizer of clays. The lime actuallyalters the ability of the clay to hold water at its surface and can react with the clay to procedure a cement which may add substantially to the strength of the lime-stabilized clay.
This paper discusses the unique phenomena which occur when lime and clay are mixed. These phenomena result because of the unique mineralogy of clays and the chemical properties of the calcium and/or magnesium compounds present int he lime.
Nature of Soil
One normally differentiates soil into groups according to particlesize. In fact, the Unified Soil Classification System divides soils into categories of gravels, sands, silts and clays, based on size. A look at the mineralogy of soils, however, shows that differentiation according to size is only a beginning in understanding soil behavior.
Soil Mineralogy
A course in soil mineralogy provides an excellent background for an engineer seeking to understand the phenomena involved in soil stabilization. The subject of soil mineralogy is complex and intricate. For our purposes, a simple comparison of the mineralogical structure of a few commonly ancountered soils will illustrate the critical role of mineralogy and crystal structure.
The Earth’s crust to a depth of about 10 miles is composed mostly of oxygen (47,3%), silicon (27,7%). These are followed by smaller amounts of the metals iron (4,5%), calcium (3,5%), sodium (2,5%), potassium (2,5%) and magnesium (2,2%). The manner in which these elements combine to from the compounds which form the soils and rock sin the crust is affected by the thermodynamics of the genesis process. Organized atomic arrangements don’t just happen but occur in a fashionwhich will preserve electrical neutrality, satisfy bonding directionality, minimize strong ion repulsions and, in short, provide the most stable arrangement of atoms possible.
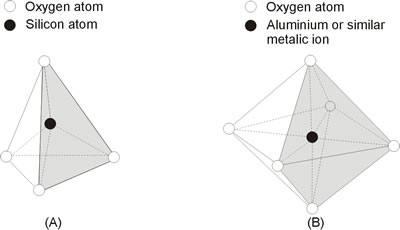
Two of the most common building blocks are shown in Figure 1. These are the silica tetrahedron and the aluminum octahodrom. Within each building block the multivalent cation is coordinated with oxygen. Thus the elements comprising approximately 83% of the arth’s crust are accounted for by these two basic building blocks.
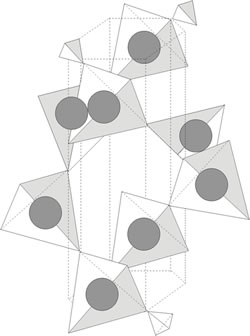
The silica tetrahedron represents the basic coordination polyhedron which forms the prolific minerals quartz and feldspar. The silica tetrahedron is not electrically neutral. Tetrahedra link together in arrangements which minimize strong repulsions between the silicon ion. The high positive charge of the silicon ion develops a variety of possible packing arrangements in response to the repulsions generated between adjacent cations. Figure 2 shows one such arrangement of these tetrahedra-the tree-dimensional space lattice silicate.
Figure 1. Two of the most common building blocks of soils: (a) silica tetrahedron and (b) aluminum octahedron.
The space-lattice silicate, or framework silicate, result when all four oxygen atoms are shared with other tetrahedra areactually grouped to form a spiral. The sharing of oxygen atoms among the tetrahedra results int he strongest type of chemical bonds among the tetrahedra-primary valence bonding.
A framework silicate like quartz is a very strong (mechanically) and durable (mechanically and chemically) mineral. This stability i sdue to the very stron internal bonding.
Another framework silicate almost identical to quartz is feldspar. Int he formation of feldspar, part of the silicon positions are filled by aluminum. Because aluminum has a lower charge by +1 than silicon, a charge imbalance within the three-dimensional structure result. This charge potential is balanced by cations adsorbed within the lattice structure such as potassium, calcium and sodium. The feldspar mineral is weaker and less durable than quartz for two reason: (1) the aluminum cation is larger than the silicon ion and does not fit among the oxygen atom sas precisely as does the silicon and (2) the adsorbed cations within the three-dimensional silicate make the structure more susceptible to weathering.
Despite the dissimilarities, both quartz and feldspar result in particles which are equi-dimensional, granular, hard and chemically relatively stable. Quartz and feldspar minerals may exist over a wide renge of particles sizes from gravel-size to silt-size. In fact quartz, due to its extremely stable nature, may even retain its mineralogical structure when weathered down to clay-sized particles (less than 2 microns in size). Ont he other hand, feldspar, because it is less mechanically and chemically stable, does not retain its basic mineralogical structure upon extreme weathering. In otherwords, one would not generally find feldspar minerals of a clay-size.
The three-dimensional framework silicates are examples of non-plastic, granular soils.
The Clay Minerals
Silica tetrahedra are joined together only at their corners because of strong repulsion between adjacent triangles of the tetrahedra. This arrangement allows the tetrahedra to form several crystal arrangements in addition to the framework silicates. Included in these structures are independent silicates, ring sor chains of tetrahedra, and sheet silicates. The sheet silicates are one unit thick but theoretically may expand infinitely int he lateral dimension. These are the units from which the clay minerals are formed.
Figure 2. Three-dimensional arrangement of silica tetrahedra typical of quartz – a stable, equi-dimensional mineral. Note the peak-to-peak coordination of tetrahedra where the bonding is vera powerfull primary valence bonding (After Moffatt et. Al., 1965).
A typical scenario for the formation of clay minerals is the chemicalweathering of feldspar. Hydrolysis is probably the most importantchemical weathering process and is caused by a reaction between the ions within the feldspar mineral and the dissociated hydrogen (h+) and hydroxyl (OH-) ions of the water.
The small h+ ions dissociated from the water molecule can easily enter the open lattice structure of the feldspar mineral and, in great concentrations, they will replace metallic ions within the lattice which have been adsorbed to neutralize the change deficiency caused by the substitution of aluminum for silicon during the formation of the feldspar. The replaced ions include sodium, potassium and calcium.
Next, the hydrogenated surfaces of the mineral become unstable, and sheets of tetrahedra and octahedra are formed and peel off. These tetrahedral and octahedral sheets are illustrated in Figure 3.
The two basic sheets, tetrahedral and and octahedral, shown in Figure 3 are stacked together to form the most prominant clay minerals. Two common arrangements of tetrahedral and octahedral sheets are presented in Figure 4. The 1:1 configuration is typical of the mineralKaolinite which has low plasticity. The 2:1 configuration is typical of the clay mineral smectite which can be very plastic and unstable.
In both configurations, a plane of atoms is common to both the tetrahedral and octahedral sheets. The bonding among the sheets is very strong primary valence bonding. However, the bonds that link the unit structures together to form particles are much weaker and are the reason for the variable response of the minerals in terms plasticity and consistency. Before the types and characterics of linkages between units can be discussed, the phenomena of surface charge of the unit cells must be explained.
During the genesis of some clay minerals a phenomena called iso-morphous substitution occurs. In this process some of the silicon ions in the tetrahedral sheet or aluminum ion sin the octahedral sheet are proced by other metallic ions of a lower positive charge (lower valence). The result is a charge deficiency which is reflected in a net negative charge at the surface of the clay unit cell. Some minerals, such Kaolinite, experience very little isomorphous substitution. In Kaolinite, about one in every 400 silicon atoms (+4 valance)., are replaced by aluminum (+3 valence) int he tetrahedral sheet. Smectite, ont he other hand, experiences abundant isomorphous substitution, about one in six aluminum atoms (+3 valance) int he octahedral sheet are replaced by magnesium (+2 valence). The result is that smectite minerals have a very hogh negative surface charge and Kaolinite minerals have a low surface charge. The ratio of surface charge between smectite and Kaolimite is about 10 to 1.
7 oldal grafikak
Figure 3. Basic sheet arrangements of (a) silica tetrahedra and (b) aluminum octahedra. Each sheet is one unit thick but may theoretically expand without bound int he lateral direction.
8 oldal grafika
Figure 4. Basic units of the 1:1 mineral Kaolinite (a) are linked with relatively strong hydrogen bonds which retain a high degree of moisture stability among layers while the basic units of the 2:1 smectite mineral (b) are linkedby weak cation attraction. The efficiency of this linkage is a function of the type and concentration of the available cations.
The manner in which the basic unit cells of the clay minerals are linked together is strongly affected by the surface charge as well as the mineral structure. Kaolinite unit cells are linked by hydrogen bonds between oxygen atom sin the base of the tetrahedral sheet and hydroxide ions at the surface of the octahedral shhet, Figure 4. Hydrogen bonding is a secondary valence bonding and is not nearly as strong as the primary valence bonds that link the sheets together. However, the bonding is strong enough to prevent the infiltration within the layers of water or foreign particles, such as cations. The result is that Kaolinite is a relatively stable clay of low plasticity.
The large amount of isomorphous substitution within smectite unitsyields a clay mineral with a substantially negative surface charge. This charge is satisfied by the adsorption of positively charged ions, cations, at the surface. Linkage between successive layers is due to the cations which balance the surface charges. This cation linkage is a very weak bonding and results in particles of clay with prominent planes of cleavage or weakness. Due to the weak inter-unit bonding, smectite clayparticles are much smaller particles than kaolinite particles.
The response of the smectite clay mineral to water is highly dependent upon the ions available int he pore water. Thus the ability to swell and shrink and demostrate plasticity is controlled by the clay-water interaction.
Clay-Water system
To this point we have established that clay particles are generally conposed of tetrahedral and octahedral sheets. Int he smectite mineral, the 2:1 arrangement, Figure 4, coupled with a substantial level of isomorphous substition, result in clay particles which have a platelike morphology. These particles are also very small and possess enormous surface area. In fact, smectite minerals often have surface areas approaching 800 m2/gm. This may be compared to only about 15 m2/gm for Kaolinite. With this enormous available surface area coupled with the highly charged nature of the clay surface, it is no wonder that the clay is so active and can so readily adsorb polar liquids like water and cations available int he enviroment.
Many explanations of the clay-water system are available. Some arequite complex. Figure 5 is an attemp to simplify the explanation while accounting for the important phenonema. First, in Figure 5a the negative clay surface is shown to be surrounded by positively charged cations attracted to the surface to equilibrate the charge potential. These ions are not orderly arranged at the clay surface but form a diffused layer. This diffusion is due to like charge repulsion and thermal agitation of the cations. A second phenomenon illustrated in Figure 5a is the diffusion of water molecules toward the high concentration of cations. The inward diffusion is triggered by the desire of nature to move to a more random (less ordered) condition.
Grafika 10 oldalrol
Figure 5. Cations and water (a dipolar melecule) are attracted to the negatively charged clay surface to satisfy the charge potential. This results in (a) adsorbed cations and water molecules and (b) a diffused layer of cations due to their thermal activity and the infusion of water toward the clay surface because of the high electrolyte concentration (After Mitchell, 1976).
The water molecules not only seek diffuse the absorbed cation layer buta re actually attracted to the cations and to the negative clay surface due to their unique dipolar structure. In Figure 5b, the water molecules are represented as molecules with distinct positive and negative sides due to the molecular arrangement of the hydrogen and oxygen atoms. Some researchers believe that a layer of water molecules is attached to the clay surface by hydrogen bonding and that subsequent layers are more loosely held. This is illustrated in Figure 5b. At anyrate, the net result is a diffused layer surrounding the clay comprised of (1) water held by hydrogen bonding and/or attracted by the diffusion gradient and (2) a diffused layer of cations, which are attracted to the negative clay surface, and anions (negatively charged ions) which are attracted to the cation and dipolar water molecules.
The net result of a highly charged surface, as is the case with smectitic clays, coupled with „unfriendly” cations, which have a single plus charge per ion and are thermally active, is a highly diffused water layer surrounding the clay particles. Because of this layer some smectites have been documented to hold seven times their dry weight in absorted water. When fully hydrated these diffused water layers force the clay platelets into a parallel arrangement which offres very little shear strength.
The thickness of the diffused water layer is greatly dependent upon the type and concentration of cations available int he pore water. Divalent cations (cations with a 2+ charge) can much more efficiently solve the negative charge potential than can monovalent cation (cations with a 1+ charge). Thus, the resulting diffused divalent cation-water layer around clay particles is much smaller than the duffused monovalent cation-water layer around clay particles of idential mineralogy.
The Dramatic Change: The Lime-Clay System
A Stable Water Layer
Lime for use in soil stabilization is most commonly produced as either hydrated high calcium lime, monohydrated dolomitic lime, calciticquicklime or dolomitic quicklime. When lime is added to a clay-water system, the divalent calcium cations yirtually always replace the cations normally adsorved at the clay surface. This cation exchange occurs because divalent calcium cations can normally replace cations of single valence, and ion sin a high concentration will replace those in a lower concentration.
The fact that calcium will replace most cations available in the water system is documented by the Lyotropic series which generally states that higher valence cations replace those os a lower valence, and larger cations replace smaller cations of the same valence. The Lyotropic series is written as: Li+ < Na+ < H+ <K+ < NH4+ << Mg++ < Ca++ << Al+++ where the cation to the right replaces the one to the left. Tgus inequal concentrations, Ca++ can easily replace the cations commonly present in most clays.
Gapon (Yong and Warketin, 1966) explains that the relationship between the cations adsorbed at the clay surface is a function of not only the concentration of cations but also the valence. Gapon’s most simple and useful equation states:
12 oldal egyenlet
where M and N are cations of valence m and n, respectively, and e refers to exchangeable and o to ions in the outside solution. The constant kdepends on the specific cation adsorption effects and upon the clay surface. Based on this equation, equal concentration of Ca++ and Ca++ ions in the outside water, natural pore water, will result in 17,5 times more Ca++ ions present at the clay surface than Na++ ions. The dual effects of divalency of the calcium ion and very high concentration which would result from the addition of lime to a soil-water system are obvious.
A New Texture
The effect of exchangeable cations on the size of the diffused water layer is illustrated in Figure 6a and 6b. Cation exchange due to the addition of lime result in stabilization of the diffused water layer and a dramatic reduction in its size. When the clay particles are allowed to approach each other more closely due to reduction in the size of the water layer an edge-to-face attraction or floccuation occurs. Flocculation is additionally enhanced due to a electrolyte concentration and high pH enviroment existing in the lime-soil-water system. The edge-to-face attraction is probably partly due to the attraction of broken bonds at the edge of the clay particles to the oppositely charged surfaces of neighboring clay particles.
The net result of cation exchange and flocculation/agglomeration of particles is:
1. Substantial reduction and stabilization of the adsorbed water layer
2. Increased internal friction among agglomerates and greater aggregate shear strength.
3. Much greater workability due to the textural change from a plastic clay to a friable, sand-like material.13 oldal a grafika
13oldal b grafikaFigure 6. The reason for the textural change is due to the phenomenon of cation exchange followed by flocculation and agglomeretion. Figure 6 (a) illustrates low strength clay soil where particles are separeted by large water layers. The addition of lime (calcium) shrinks the water layer (b) allowing the plate-like particles to flocculate.
Immediate Strength Improvement
Laboratory evidence substantiates textural and property changes due to cation exchange followed by flocculation/agglomeration. Table 1 illustrates the ability of relatively small percentages of lime to reduce the plasticity index and swell potential of plastic, troublesome clays to innocuous levels. Figure 7 illustrates the increase in shear strength, as measured by the California Bearing Ratio (CBR) of a low plasticity clay due to textural changes in the clay. These textural changes are due to cation exchange followed by flocculation/agglomeration, and these changes essentially as occur as rapidly as the lime can be intimately mixed with the clay. The soaked CBR tests (96 hour soak) in Figure 7 were performed immediately after compaction, without long-term cure effects.
Table 1. Attemberg Limits for Natural and Lime-Treated Soils (After Thompson, 1967).
14 oldal táblázat
The reason for the improved shear strength is illustrated in Figure 6 where an unaltered clay whose diffused water layer is hydrated is compared to the same clay after lime stabilization. The structure of the clay platelets surrounded by the hydrated, diffused water layers provedes very little shear strength. The only resistance to relative movement is due the overlapping and interferences among the water layers. On the other hand, in the flocculated structure, the summation of the edge to face contacts provides a more substantial sear strength.
Long Term Strength
The phenomenon of cation exchange and the concomitant textural changes occurs with all clays. Of course, the degree of effect and/or amount of lime rquires to cause cation exchange is based on the chemical and mineralogical conditions of the soil and the water environment.
15 oldal grafikon
Figure 7. CBR-Moisture content relations for natural and lime-treated (3% 5%) CL Soil (AASHTO T compaction) (After Thompson, 1970).The long-term strength is more complex and is heavily influenced by soil conditions and mineralogical properties. However, many clay soils arepozzolanically reactive when stabilized with lime and respond with anappreciable strength gain due to the development of a cemented matrix among the soil particles.
A pozzolan is defined as a finely divided silicions or aluminous material which in the presence of water and calcium will form a cemented product. The cemented products are calcium-silicate-hydrates and calcium-aluminate-hydrates. These are same hydrates that form duringthe hydration of Portland cement.Clay is a pozzolan as it is a source of silica and alumina for the pozzolanic reaction. Clay-silica and clay-alumina become soluble or available in a high pH enviroment, Figure 8. The pH of water saturated with lime is 12.45. Thus a soil-lime-water system has a pH hogh enough to solubilize silica and alumina for pozzolanic reaction. As long as enough residual calcium remains in the system to combine with the clay-silica and clay-alumina and as long as the pH remains high enough to maintain solubility, the pozzalanic reaction will continue. The reaction is illustrated by the following equations:
Ca++ OH- + Soluble Clay Silica – Calcium Silicate Hydrate (CSH)
Ca++ OH- + Soluble Clay Alumina – Calcium Aluminate Hydrate (CAH)Eades and Grim (1966) skillfully adopted the pH increase phenomenon in a design procedure for lime-soil mixtures. Their procedure requires for succifient lime to be added to the soil to satisfy all immediately occuring reactions, and yet provide enough residual lime to maintain a pH of 12,4 for sustaining the strength-producing reaction.
What is unique about the pozzolanic phenomenon is the cooperative reaction between the lime and the clay. The lime induces the high pH environment which solubilizes the silica and alumina. The lime also provides the residual free calcium which combines with the silica and alumina supplied by the clay to produce the pozzolanic reaction.
Evidence of the strength of the pozzolanic reaction comes from both field and laboratory data. Strength increases of greater than 100 psi can be achieved with many soils following 28 day curing at temperatures of approximately 70oF. Extended curing either in the laboratory or under field conditions may produce strength increases of several hundred psi.
Field data indicate that with some soil-lime mixtures strength continues to increase with time up to in excess of ten years.The Mohr-Coulomb criteria is often used to evaluate the shear strength of granular materials. This criteria states that shear strength is provide by (1) the cohesive strength of the soil and (2) the strength due to internal friction. Mathematically the law reads:
Shear Strength = Cohesive Strength © + Normal Stress (N) x tan Ø
Where Ø is the angle of internal friction.17 oldal grafika
Figure 8. Effect of pH on the solubility of clay silica and clay alumina (After Keller, 1964).
Typical angles of shearing resistance or internal friction for lime stabilized clays are between 25o F and 35o F. This may be as much as 100% higher than for the natural clay soil . This component then adds to the shear strength more as the confining pressure ont he soil increases. The cohesion valum (c) increases with the conpressive strength of the mixture. A rough estimate of c is 30 percent of the unconfined compressive strength (Little, et. Al.,1987).
Stiffness and load spreading capability of the stabilized clay also increase with development of pozzolanic strength. Field deflection data recorded by Texas A&M University has shown that resilient moduli of plastic Texas clays in the Houston area have been increased from between 5,000 and 10,000 psi for the natural clay to between 20,000 psi and 70,000 psi after lime stabilization. Other researchers have documented stiffnesses of over 100,000 psi for lime stabilized clays (TRB Crcural, 1976).
Suitable Soils for Lime Stabilization
Experience has shown that lime will react with medium, moderately fine and fine-grained soils to produce decreased plasticity, incrreased workability, reducer swell and increased workability,reduced swell and increased strength. Generally speaking, those soils classified by the Unified System as CH, CL, MH, SC, SM, GC, SW-SC, SP-SC ,SM-SC, GP-GC, GM-GC arepotentially capable asbeing stabilized with lime.
The key to a pozzolanic reaction resulting in long-term strength gain is the presence of a reactive clay to provide the pozzolans. Although lime cannot react pozzolanically whit sands (composed of framework silicates9 whitch have no clay fraction, lime may be an effective stabilizer with sandy or silty soils which have a clay content as low as sever percent and a plasticity index as low as 11(Little,et,al.,1987).
As a general guide to stabilization (Little,et.al.,19879 proposed that lime stabilization should be considered as the primary stabilizer or at least as a pre-stabilizer for all soils having plasticity indices of greater that 10 and greater that 25 percent of the shoil smaller that the number 200 sieve. In the case of plasticity indices above 30 and greater that 25 percent material passing the number 200 sieve, the selection criteria recommended by Litle, et. al. (1987) for use by the Air Force requires the use of lime or lime as a pre-treatment to reduce the plas ticity index below 30 followed by Portland cament stabilization.
The extent which the soil-lime pozzolanic reaction proceeds is influenced primarily by natural soil properties. With some soils, the pozzolanic reaction is inhibited, and cementing agents are not extensively formed. Those soils that react with lime to produce substantial strength increase (greater than 50 psi following 28 day curing at 73o F )are ”are ”reactive” and those that disply limited pozzolanic reactivity (less than 50 psi strength increase) are ”nonreactive” (Thompsom, 1970).
The majorsoil properties and characteristics which influence the lime-reactivity of a soils, i.e.,ability of the soil to react with lime to produce cementitious materials, are soils pH, organic carbon content, natural drainage, presence of excessive quantitites of exchangeable sodium ,clay mineralogy ,degree of weathering ,presence of carbonates,extractableiron, silica-sesquioxide ration and silica-aluminal ratio.Itis emphasized that the main factors controlling the development of pozzolanic cementing in a lime treated soil are the inherent properties and characteristics of the soil. If a soil is ”nonreactive”, extensive pozzolanic strengthdevelopement wil not be achieved regardless of lime type, lime percentage or curing conditions of time and temperature.
Soil-lime reactions are complexand not completely understood at this time. However, sufficient basic understanding and successful field experience are available to provide the basis of an adequate technologyfor successfully utilizing soil-lime stabilization under a wide variety of conditions.
Thompson (1970)has published data, Table 2, which demonstrates the wide-randing pozzolanic reactivity which can occur with fine-grained soils.
Table 2. Compressive Strength Data for Natural and Lime-Treated Soils (After Thompson, 1970).
19 oldal tablazat